The assembly of the T3SS
|
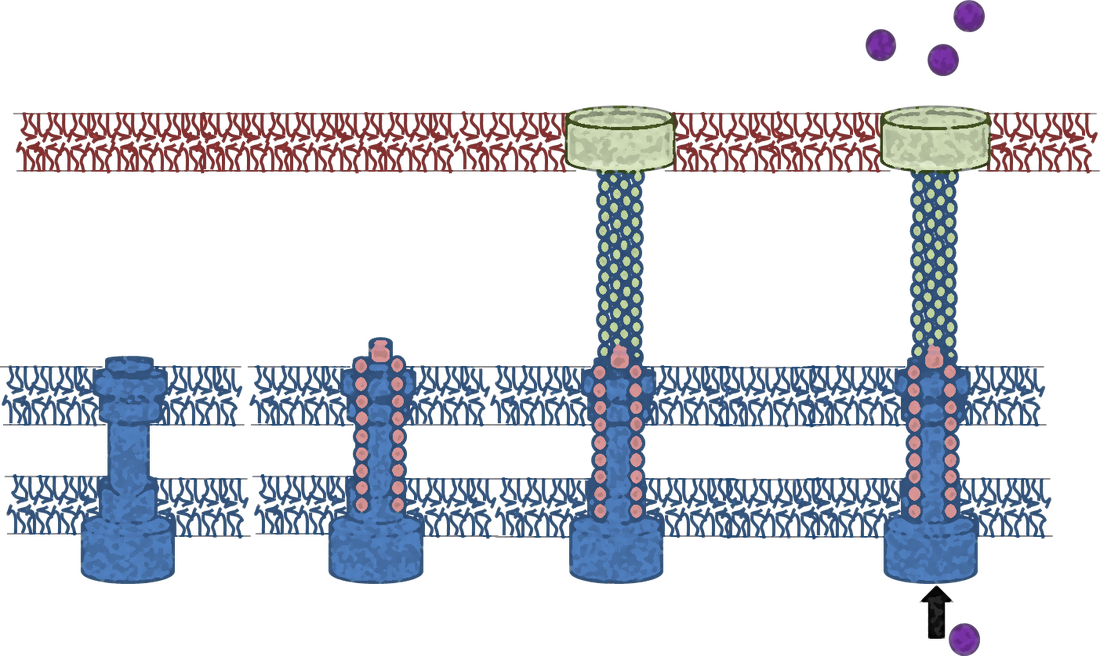
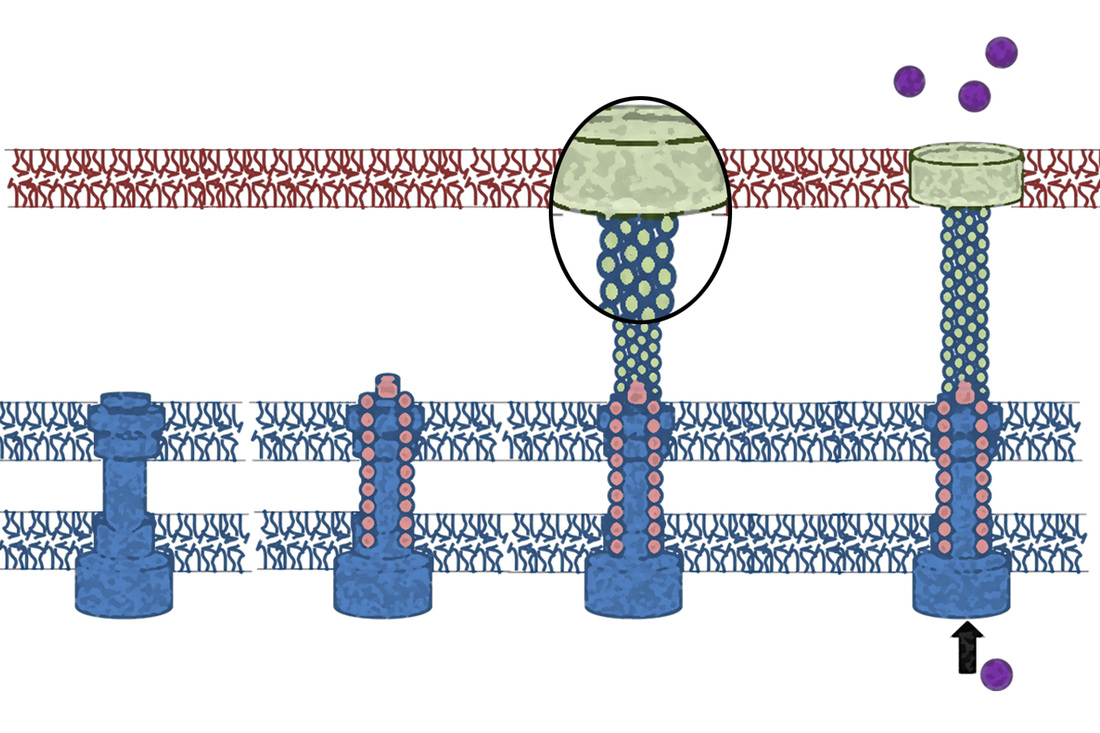
The T3SS is composed of about 20 different proteins that are expressed and assembled only under specific conditions that are similar to those existing in the human gastrointestinal tract, such as temperature, pH, and physiological osmolarity. Understanding the fundamental processes of the T3SS will allow us to rationally modify and block the activity of this widely conserved virulence machinery employed by Gram-negative bacterial pathogens for numerous applications in biotechnology and medicine.
|
Host membrane targeting
|
The first bacterial component to physically interact with the host cell membrane is known as the translocon complex. The complex, which is made up of 2-3 proteins, facilitates the transport of effectors across the host-cell plasma membrane. Despite the critical role of the translocon in bacterial virulence, the mechanisms of translocon assembly, interactions with the host membrane, and pore formation activity remain to be elucidated.
|
Transmembrane domains within the T3SS
|
Although many of the proteins that form the T3S apparatus are transmembrane (TM)- or membrane-associated proteins, our knowledge of these proteins is limited mainly to their soluble domains. In recent years, considerable data have accumulated regarding the causal involvement of the TM domains in the assembly and regulation of membrane complexes, especially receptor complexes. Oligomerization of TM domains is known to be extensively involved in the function of many proteins, and it is not therefore surprising that deficient oligomerization accounts for a variety of diseases, ranging from cancer to amyloidal diseases. We study the role of the TM domains of different T3SS components and their involvement in the assembly and regulation of the T3SS, and we seek to design novel inhibitors to block these TM-TM interactions.
|